
The Development of Medical Robots
According to Winter Green Research, the surgical robot market was valued at $3.2 billion in 2014. North America remains the largest market, driven by increased government funding, healthcare system reforms, and growing awareness of minimally invasive surgery. However, the focus is gradually shifting towards Asia as these factors gain momentum.
With the introduction of next-generation systems, surgical robots are moving from large-scale open surgeries to more precise, smaller procedures. The global surgical robot market is projected to reach $20 billion by 2021, signaling a significant growth trajectory.
In recent years, surgical robots have made remarkable progress. As the robotics industry expands, medical robots are gaining increasing attention worldwide. In the U.S., research is focused on surgical treatment machines, prosthetic robots, rehabilitation robots, psychological assistance robots, personal care robots, and intelligent health monitoring systems.
Europe has also taken steps to promote medical robotics through the "Robotics for Health-care" network, aiming to boost innovation and application within the region.
Surgical robots, a key branch of medical robotics, have seen rapid development over the past decade. Current research focuses mainly on surgical, rehabilitation, nursing, and service robots.
Why does the Da Vinci surgical robot dominate the global market? Known as the "Endoscopic Surgical Instrument Control System," it was developed and marketed by Intuitive Surgical, founded in 1995 in Sunnyvale, California.
As the most renowned surgical robot today, the Da Vinci system represents minimally invasive surgery. Its precision, minimal trauma, quick recovery, and remote guidance capabilities make it highly popular among surgeons and patients alike.
Currently, the Da Vinci system is widely used in general surgery, urology, cardiothoracic surgery, gynecology, ENT, and pediatric surgery. It is particularly common in prostatectomy and increasingly used in heart valve repair and gynecological procedures.
As of October 2017, there were 67 Da Vinci surgical robots in mainland China, despite the lower number compared to other countries, the utilization rate per unit is the highest globally.

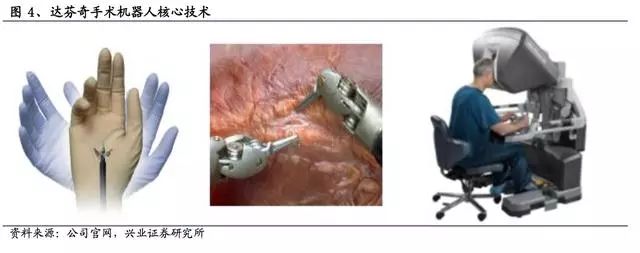
Core Technologies of the Da Vinci Surgical Robot
The Da Vinci surgical robot represents the pinnacle of current surgical robotics. It features three key technologies: the free-moving arm wrist (Endo Wrist), 3D high-definition imaging, and an intuitive console interface for human-computer interaction.
Domestic Surgical Robot Market and Current Status
Despite limitations in installed capacity, the surgical penetration rate in emerging markets like China is still low. However, the domestic medical robot market holds great potential.
China's aging population and rising incidence of stroke and trauma-related disabilities are increasing demand for minimally invasive, efficient, and high-quality medical services. This growing need is expected to drive rapid market growth in China.
Sun Lining, deputy director of the State Key Laboratory of Technology and Systems and chairman of Bosch Robot, noted that the development of medical robots faces many challenges, including difficulties in technology transfer and long industrialization paths. However, he also highlighted that policy support and capital inflow are now beginning to make a difference.
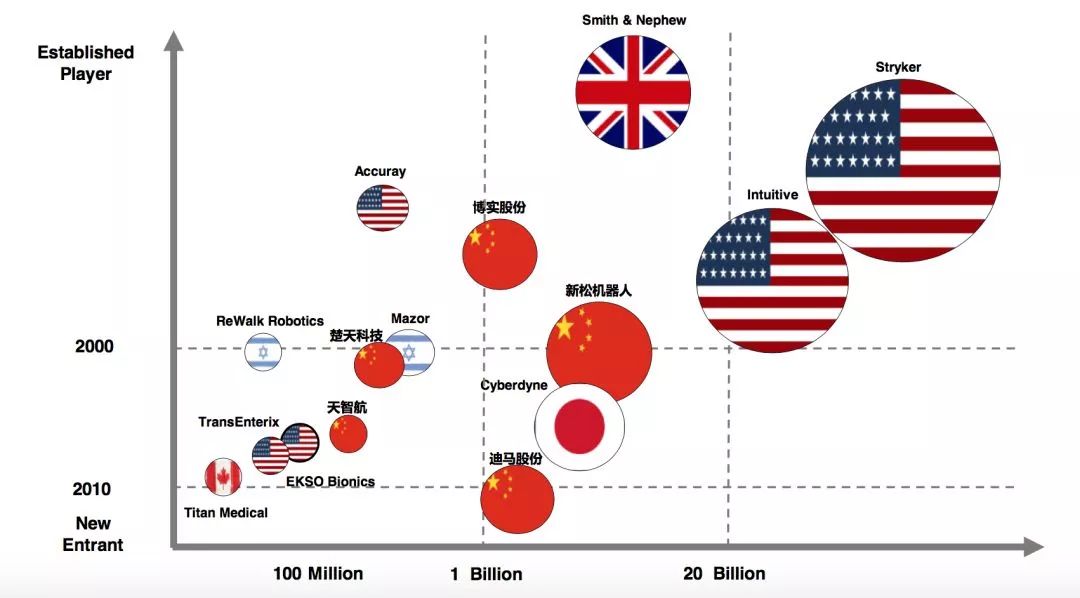
From Experimental Products to Industrialization
According to a joint report by PricewaterhouseCoopers and ROBO Medical, American companies still dominate the global medical robot market. While many entrepreneurs have entered this field in recent years, the Chinese surgical robot market remains in its early stages.
China's surgical robots started later than those of foreign countries. Most companies with strong market presence are research-based, with R&D teams forming startups, raising capital, and solving industrialization challenges before entering the market.
In 1997, CRAS, a brain surgery robotic assistive system developed by Beijing University of Aeronautics and Astronautics, Tsinghua University, and the Navy General Hospital, performed the first robotic minimally invasive surgery. In 2000, Tianjin Huazhi acquired the technology, and in 2002, the product received CFDA approval.
In 2017, Wang Rongjun established Huazhi Mini-Chuang, which later acquired Tianjin Huazhi in 2017, holding 51% equity.
In 2013, Harbin Institute of Technology's minimally invasive abdominal surgery robot system achieved independent intellectual property rights, breaking Da Vinci's technological monopoly. Sizherui Medical was established upon completion of the research. In May 2015, Bosch Co., Ltd. invested 20 million RMB to subscribe to Sizherui Medical's newly issued capital.
Beijing TINAVI Medical Technology Co., Ltd., established in 2005, developed orthopedic robots based on results from the 863 Project. It obtained a product registration license in 2010 and was listed on the New Third Board in 2015.
“Moving from experimental products to usable ones is a painful process,†said Wang Rongjun. “Academic researchers often lack commercialization skills. My professor was willing to do this.†Compared to TINAVI and Bosch, Huazhi Microcred’s commercialization came later. In February 2018, Huazhi Microcred secured 50 million yuan in its first round of financing.
Over the past decade, many technical barriers have hindered the transition from R&D to commercialization. For example, developing a robotic arm for minimally invasive neurosurgery took about seven or eight years, with 100% localization achieved.
Zhu Gang, a urology chief physician at Beijing United Family Hospital, noted that Da Vinci’s robotic arms are high-value consumables, costing tens of thousands of yuan each after 10 uses. This makes them expensive compared to foreign manufacturers.
Localization brings cost advantages. Wang Rongjun stated that the accuracy of domestic robotic arms is comparable to foreign products. While Da Vinci’s neurosurgery robot costs around ten million, Huazhi’s costs about one million.
“Rural Encircling the Cityâ€
When Hua Zhi raised its first round of financing, the biggest concern was the safety and stability of the technology. “After passing over 40,000 clinical cases, the product’s security and stability have been proven by the market,†said Wang Rongjun.
Director Lu Wangsheng of Beijing Tiantan Puhua Hospital explained how frameless stereotactic technology solves traditional surgery issues, such as anesthesia problems and thin skull bones in children, making procedures safer and more accessible.
Top hospitals prefer foreign equipment due to concerns about safety and stability. How can domestic companies like Huazhi enter hospitals? Localization helps reduce costs, enabling second- and third-tier hospitals to adopt surgical robots.
Huazhi adopts a strategy of “encircling the city from the countryside.†While it may not enter top hospitals directly, it targets prefecture-level cities, where resources are limited and doctors can operate independently, reducing labor costs.
Large-scale profits come not just from selling devices but from consumables and follow-up services. According to Wang Rongjun, hospitals buy both equipment and post-service. Data from Hua Zhi’s business plan shows a declining trend in equipment sales and an increasing trend in consumables sales since 2018.
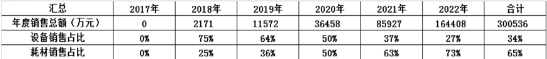
Huazhi Mini Creation Annual Sales Plan
“Training in primary hospitals allows doctors to perform biopsies and tumor removal after learning cerebral hemorrhage treatment, helping to sink medical resources. We have expert teams that act as seeds, capable of rooting in local areas,†said Wang Rongjun.
He emphasized that success depends on solving user pain points and providing value-added services. If both are met, the product can sustain long-term use.
Comparing China’s cardiovascular bypass industry over the past 40 years, Wang Rongjun believes the surgical robot industry will see major changes in the next few years. Minimally invasive surgery is similar to the adoption of heart stents, offering faster recovery and cost-effective treatment from an insurance perspective.
However, surgical robots face two levels of safety certification and clinical trials. Without proof of safety and effectiveness, they cannot obtain certification or enter clinics. This is why most industry leaders are deeply integrated with universities and hospitals, leading to a slow but steady growth phase. Latecomers must overcome the challenge of time to succeed.
usb type c cable,4-in-1 data cable,type-c charger,type-c charging cable
DongGuan BoFan Technology Co.,Ltd. , https://www.ufriendcc.com